
Layers of backbone in the core of the structure is a feature that many, but not all, globular proteins have. Layers of Backbone Present in the Structure The objective of this page is to introduce the tertiary structures of globular proteins by illustrating these characteristics of globular proteins. Other important characteristics in the absence of backbone layers are the presence of disulfice bonds, of chelated metal ions or of intrinsically unstructured segments. The tertiary structure of many globular proteins can be characterized by the number of layers of peptide backbone which are present and the attractive forces which are generated by these layers. Hemoglobin is a good example of a protein that has a quarternary structure. Some globular proteins have a quaternary structure, and it is formed when two or more globular protein molecules (monomer) join together and form a multimeric unit. Links to sites having structures that illustrate disulfide bonds are at Cystine. The Disulfide bond is the one type of covalent bond that can play an important role in maintaining the tertiary structure as well as connecting two or more peptide chains together.
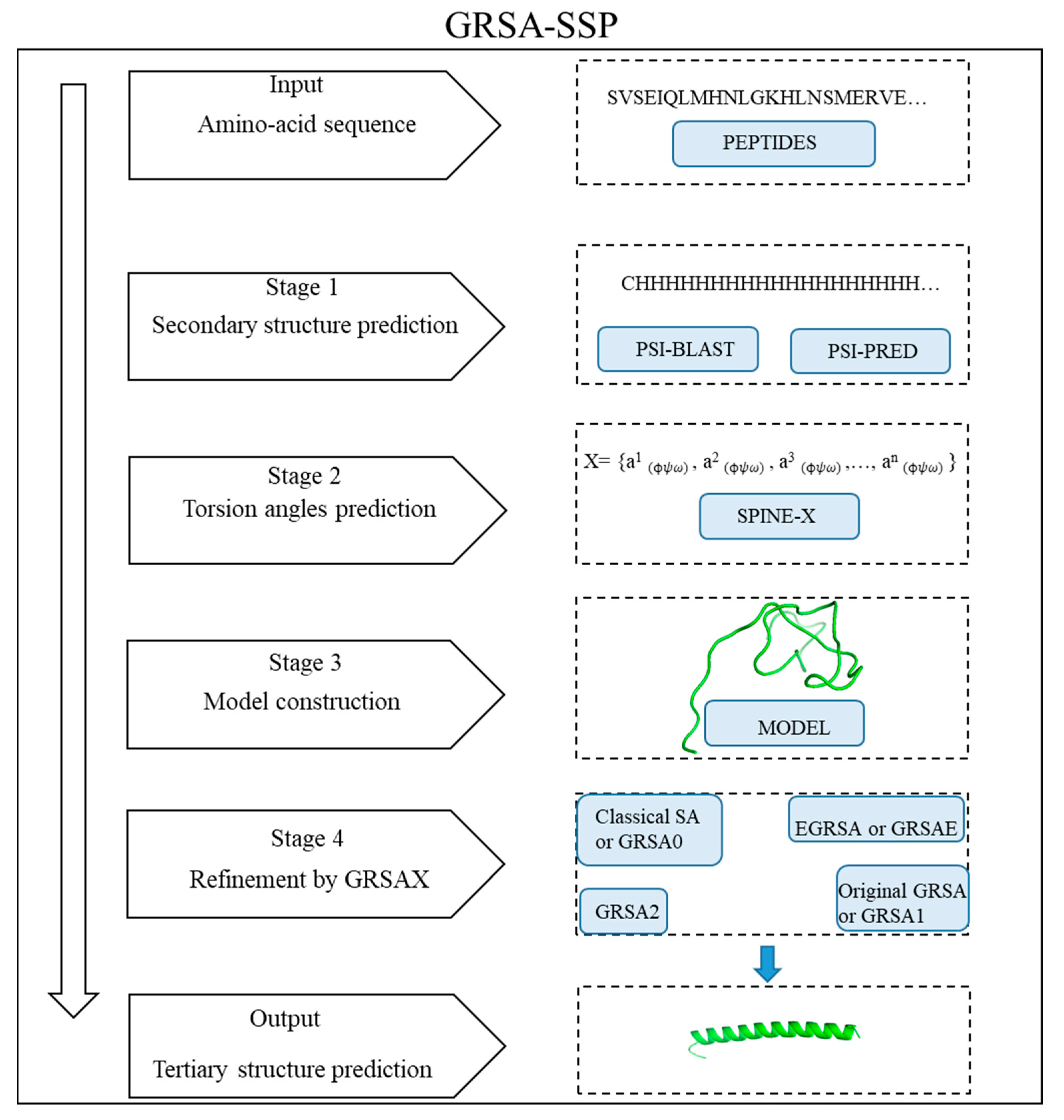
The attractive forces of salt bridges are important in maintaining some tertiary structures, but they also can be involved in the binding of ligands. Examples of finding and visualizing both types in globular proteins are at hydrogen bonds. Hydrogen bonds between back bone atoms are important in maintaining secondary structures, and those between side chains are involved in maintaining the tertiary structure. For the most part, these attractions are between the atoms of the side chains but can be between the side chains and a bound ligand. Non-covalent molecular attractions are important forces in maintaining the folded conformation of a globular protein. The tertiary structure is the overall 3D structure of a globular protein and is produced by folding the helices and sheets upon themselves with turns and loops forming the folds. Turns are classified as a secondary structure even though their structures are ordered but not repetitive.

Helices, β-sheets and turns are three important types of secondary structures. The peptide chain can be folded in an ordered and repetitive fashion, and the structures with ordered and repetitive conformations are called secondary structures.
The primary structure is simply the sequence of amino acids forming the peptide chain. Usually the structure of a globular protein is divided into three or four levels. Globular proteins have a 3D molecular structure that has a shape that is anywhere from a sphere to a cigar.


 0 kommentar(er)
0 kommentar(er)
